Current Challenges in Clinical Research that Hamper Trials

Clinical trials have existed for a long time but they became even more important when COVID-19 raged. Traditionally, all vaccines, medical devices, and beneficial drugs designed for specific diseases are created by carrying out intensive tests to ascertain their safety and viability in treating the disease through clinical trials. The process of a clinical trial can be excruciatingly long and laborious with several factors that could deter its progress and success. We will discuss the notable challenges common to clinical research, how it affects the process and the results of clinical trials, how sponsors and Principal Investigators (PIs) leave crucial issues out unsorted, and how to best take advantage of patient identity verification.

Preventing professional patients is possible with RightPatient.
Current challenges in clinical research
Arduous, dangerous, time-intensive, and complex are the words that can fully capture the nature of the process that surrounds clinical trials. The trial is supervised by Sponsors and PIs to ensure that there are no violations of the rules and regulations to the letter such as the enrollment of the right amount of patients that fit the required conditions for the trials. They are also tasked with the stringent management of several trial sites. Here are some of the challenges that oppose the success of clinical trials.
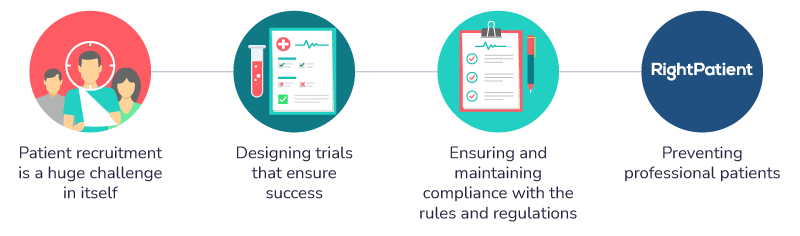
Patient recruitment can pose a huge challenge
The most recurrent aspect in the list of current challenges in clinical research that often occurs right from the conception of the idea of a clinical trial is the issue of patient recruitment. Some of the problems, in this case, include the unresponsiveness of patients, the attraction of patients with conditions that do not fit the subject of the test, or poorly performing research sites. These could end the clinical trial before it even starts. If we are to delve into the lengthy list of the challenges of patient recruitment, it would take an entirely different article of its own.
The focus here is that there can be no clinical trial if test subjects are not available or they do not fit the criteria for the trial. The problems that may arise from the trials may result from the fact that research data was not enough to affirm the drug/vaccine’s effectiveness. Irrespective of the promising nature of the agent, the drug may fail to progress to the subsequent phases necessary for approval for general use.
Designing trials that ensure success
The process of designing a successful clinical trial is also one of the top challenges because it has to satisfy everyone. At the start, it was not so complex, all rules and regulations were often in their infancy, and things were always pretty easy.
Current challenges in clinical trials – RightPatient addresses the overlooked one.
Modern clinical trials, however, have taken on a new shape of complexity with rules that must be adhered to from top to bottom. It must be simple for patients to understand and obey, it must proffer answers to rather difficult questions in the right way, and ultimately, it must satisfy the necessary stakeholders. Meeting expectations in a trial design is not easy. This makes it one of the most consistent of the current challenges in clinical research.
Ensuring and maintaining compliance with the rules and regulations
The healthcare industry is a highly monitored sector because of the gravity of the healthcare outcomes of patients in the system. The subsequent products of clinical trials such as drugs, vaccines, treatment processes, and medical devices represent outcomes, they are also subject to heavy regulations.
The existence and importance of the regulations are relateable but it also makes for a herculean task in strict compliance. The slightest discrepancy could hinder the trial and lead to a huge financial loss running up to millions. Maintaining and ensuring compliance remains a great challenge with unlimited imposed regulations.
Preventing professional patients
Professional patients is not a commonly discussed term whenever issues related to current challenges in clinical research are raised. Nonetheless, it is also a crucial issue. It goes by different terms like “professional study subjects” and “duplicate study subjects”, and they are individuals who are capable of thwarting the credibility of clinical trials. They are culpable for participating in multiple trials simultaneously or consecutively, thereby influencing ruining the overall results of the trials that follow.
A relevant illustration is that of a duplicate study subject that has been diagnosed with a heart condition and has participated in a trial and received dosages of an experimental drug. The subject then goes almost immediately to partake in another trial. The problem lies in the fact that the initial drug is still in their system and it will project wrongly on the second trial. There is also the danger attached to going to multiple trials as it will not only skew the results of the trials but will also be harmful to them.
These types of patients affect the integrity of clinical trials while also presenting a danger to their health. In addition, they could lead to losses worth millions and can lead to experimental agents being deemed as failures because of skewed results. Fortunately, RightPatient can prevent
If you are looking for the right tool to help in dealing with professional patients in clinical trials, you can count on RightPatient. It is a trusted touchless patient identification platform that has earned great reviews from top healthcare providers. It has ample capabilities and experience that could put an end to issues of professional patients effectively. The platform could help to save millions worth of losses, and mitigate delays in approvals, and enhancing the integrity of trials. RightPatient is the perfect way to prevent professional study subjects in clinical trials.





























 Michael Trader is President and Co-Founder of RightPatient®. Michael is responsible for overseeing business development and marketing activities, government outreach, and for providing senior leadership on business and policy issues.
Michael Trader is President and Co-Founder of RightPatient®. Michael is responsible for overseeing business development and marketing activities, government outreach, and for providing senior leadership on business and policy issues.